is sublimation a physical or chemical change

Sublimation is the transition of a substance direct from the solid to the gas land,[1] without passing through the liquid land.[ii] Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple betoken in its phase diagram, which corresponds to the lowest pressure at which the substance can exist every bit a liquid. The reverse procedure of sublimation is deposition or desublimation, in which a substance passes directly from a gas to a solid phase.[three] Sublimation has also been used as a generic term to draw a solid-to-gas transition (sublimation) followed by a gas-to-solid transition (deposition).[iv] While vaporization from liquid to gas occurs as evaporation from the surface if information technology occurs below the boiling signal of the liquid, and as boiling with germination of bubbles in the interior of the liquid if it occurs at the boiling point, at that place is no such distinction for the solid-to-gas transition which always occurs as sublimation from the surface.
At normal pressures, well-nigh chemical compounds and elements possess three different states at different temperatures. In these cases, the transition from the solid to the gaseous land requires an intermediate liquid state. The pressure referred to is the partial pressure of the substance, not the total (east.g. atmospheric) pressure of the unabridged system. So, all solids that possess an observable vapour pressure at a certain temperature usually can sublime in air (eastward.g. water ice just below 0 °C). For some substances, such as carbon and arsenic, sublimation is much easier than evaporation from the melt, because the force per unit area of their triple point is very high, and it is difficult to obtain them as liquids.
The term sublimation refers to a physical change of state and is not used to depict the transformation of a solid to a gas in a chemical reaction. For example, the dissociation on heating of solid ammonium chloride into hydrogen chloride and ammonia is non sublimation but a chemical reaction. Similarly the combustion of candles, containing paraffin wax, to carbon dioxide and water vapor is non sublimation but a chemical reaction with oxygen.
Sublimation is caused past the absorption of heat which provides plenty free energy for some molecules to overcome the bonny forces of their neighbors and escape into the vapor stage. Since the process requires boosted energy, information technology is an endothermic change. The enthalpy of sublimation (also called heat of sublimation) can be calculated by calculation the enthalpy of fusion and the enthalpy of vaporization.
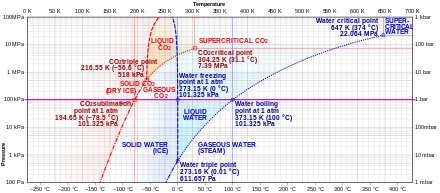
Comparison of phase diagrams of carbon dioxide (reddish) and water (blue) showing the carbon dioxide sublimation signal (middle-left) at i atmosphere. Every bit dry ice is heated, information technology crosses this point along the bold horizontal line from the solid phase straight into the gaseous phase. Water, on the other hand, passes through a liquid phase at 1 temper.
Examples
Carbon dioxide

Solid carbon dioxide (dry ice) sublimes everywhere along the line below the triple point (e.g., at the temperature of −78.5 °C (194.65 1000, −109.30 °F) at atmospheric pressure, whereas its melting into liquid COtwo tin can occur along the solid-liquid line at pressures and temperatures above the triple point (i.due east., 5.1 atm, −56.half dozen °C).
Water
Snow and ice sublime, although more slowly, at temperatures below the freezing/melting indicate temperature line at 0 °C for partial pressures below the triple point pressure of 612 Pa (0.00604 atm).[5] In freeze-drying, the material to be dehydrated is frozen and its water is immune to sublime nether reduced pressure or vacuum. The loss of snow from a snowfield during a common cold spell is oftentimes caused by sunshine interim directly on the upper layers of the snow. Ablation is a process that includes sublimation and erosive habiliment of glacier ice.[ citation needed ]
Naphthalene
Naphthalene, an organic compound commonly institute in pesticides such equally mothballs, sublimes easily considering it is made of not-polar molecules that are held together merely by van der Waals intermolecular forces. Naphthalene is a solid that sublimes at standard atmospheric temperature[6] with the sublimation point at around 80°C or 176°F.[vii] At low temperature, its vapour pressure is high enough, onemmHg at 53°C,[8] to brand the solid form of naphthalene evaporate into gas. On cool surfaces, the naphthalene vapours will solidify to class needle-like crystals.

Experimental set up for the sublimation reaction of naphthalene Solid naphthalene sublimes and form the crystal-like structure at the bottom of the watch glass

Solid compound of naphthalene sublimed to form a crystal-like structure on the cool surface.
Other substances

Camphor subliming in a cold finger. The crude product in the bottom is nighttime brownish; the white purified product on the bottom of the common cold finger above is difficult to encounter against the light groundwork.
Iodine produces fumes on gentle heating, although this is above the triple betoken and therefore not true sublimation. It is possible to obtain liquid iodine at atmospheric pressure level past controlling the temperature at just above the melting indicate of iodine. In forensic science, iodine vapor tin reveal latent fingerprints on paper.[9] Arsenic can as well sublime at high temperatures.
Cadmium and zinc are not suitable materials for use in vacuum because they sublime much more other common materials.[ citation needed ]
Purification past sublimation

Crystals of ferrocene after purification by vacuum sublimation
Sublimation is a technique used by chemists to purify compounds. A solid is typically placed in a sublimation apparatus and heated under vacuum. Under this reduced pressure, the solid volatilizes and condenses as a purified compound on a cooled surface (cold finger), leaving a non-volatile rest of impurities behind. Once heating ceases and the vacuum is removed, the purified compound may be nerveless from the cooling surface.[10] [11] For even higher purification efficiencies, a temperature gradient is applied, which also allows for the separation of unlike fractions. Typical setups utilize an evacuated drinking glass tube that is heated gradually in a controlled manner. The material flow is from the hot end, where the initial material is placed, to the cold end that is connected to a pump stand. Past controlling temperatures forth the length of the tube, the operator tin can control the zones of re-condensation, with very volatile compounds being pumped out of the system completely (or defenseless by a separate cold trap), moderately volatile compounds re-condensing along the tube according to their dissimilar volatilities, and non-volatile compounds remaining in the hot end. Vacuum sublimation of this blazon is too the method of choice for purification of organic compounds for use in the organic electronics industry, where very loftier purities (frequently > 99.99%) are needed to satisfy the standards for consumer electronics and other applications.[ citation needed ]
Historical usage
In ancient alchemy, a protoscience that contributed to the evolution of modernistic chemistry and medicine, alchemists developed a structure of basic laboratory techniques, theory, terminology, and experimental methods. Sublimation was used to refer to the process in which a substance is heated to a vapor, then immediately collects every bit sediment on the upper portion and neck of the heating medium (typically a retort or alembic), but can also be used to describe other similar non-laboratory transitions. It was mentioned past alchemical authors such as Basil Valentine and George Ripley, and in the Rosarium philosophorum, as a process necessary for the completion of the magnum opus. Here, the word sublimation was used to draw an substitution of "bodies" and "spirits" like to laboratory phase transition between solids and gases. Valentine, in his Le char triomphal de 50'antimoine (Triumphal Chariot of Antimony, published 1646) made a comparison to spagyrics in which a vegetable sublimation tin exist used to separate the spirits in wine and beer.[12] Ripley used language more indicative of the mystical implications of sublimation, indicating that the procedure has a double aspect in the spiritualization of the body and the corporalizing of the spirit.[13] He writes:[14]
And Sublimations we make for three causes,
The first cause is to brand the body spiritual.
The second is that the spirit may exist corporeal,
And become fixed with it and agnate.
The 3rd cause is that from its filthy original.
It may be cleansed, and its saltiness sulphurious
May be diminished in information technology, which is infectious.
Sublimation predictions
The enthalpy of sublimation has usually been predicted using the equipartition theorem. If the lattice energy is assumed to exist approximately one-half the packing energy,[ clarification needed ] so the following thermodynamic corrections tin exist applied to predict the enthalpy of sublimation. Assuming a i tooth ideal gas gives a correction for the thermodynamic environment (pressure level and book) in which pV = RT, hence a correction of 1RT. Additional corrections for the vibrations, rotations and translation then need to be applied. From the equipartition theorem gaseous rotation and translation contribute ane.5RT each to the terminal state, therefore a +3RT correction. Crystalline vibrations and rotations contribute 3RT each to the initial state, hence −6RT. Summing the RT corrections; −6RT + 3RT + RT = −2RT.[15] This leads to the following judge sublimation enthalpy. A similar approximation can be plant for the entropy term if rigid bodies are assumed.[sixteen] [17]
Dye-sublimation printing
Dye-sub press is a digital printing engineering using total color artwork that works with polyester and polymer-coated substrates. Likewise referred to as digital sublimation, the process is commonly used for decorating dress, signs and banners, besides equally novelty items such as prison cell phone covers, plaques, coffee mugs, and other items with sublimation-friendly surfaces. The process uses the science of sublimation, in which heat and pressure level are applied to a solid, turning it into a gas through an endothermic reaction without passing through the liquid stage.[ commendation needed ]
In sublimation press, unique sublimation dyes are transferred to sheets of "transfer" paper via liquid gel ink through a piezoelectric print head. The ink is deposited on these loftier-release inkjet papers, which are used for the next pace of the sublimation printing procedure. After the digital design is printed onto sublimation transfer sheets, it is placed on a oestrus press forth with the substrate to be sublimated.[ citation needed ]
In order to transfer the image from the paper to the substrate, it requires a oestrus press process that is a combination of fourth dimension, temperature and pressure. The heat press applies this special combination, which can change depending on the substrate, to "transfer" the sublimation dyes at the molecular level into the substrate. The almost mutual dyes used for sublimation activate at 350 degrees Fahrenheit. Still, a range of 380 to 420 degrees Fahrenheit is normally recommended for optimal color.[ citation needed ]
The stop result of the sublimation process is a nearly permanent, high resolution, full colour impress. Because the dyes are infused into the substrate at the molecular level, rather than applied at a topical level (such as with screen printing and straight to garment printing), the prints volition not crack, fade or peel from the substrate under normal conditions.[ citation needed ]
See also
- Ablation
- Enthalpy of sublimation
- Freeze-drying
- Freezer fire – mutual process involving sublimation
- Phase diagram
Table
| To From | Solid | Liquid | Gas | Plasma |
|---|---|---|---|---|
| Solid | Melting | Sublimation | ||
| Liquid | Freezing | Vaporization | ||
| Gas | Degradation | Condensation | Ionization | |
| Plasma | Recombination |
References
- ^ "Sublimate". Merriam-Webster Dictionary.
- ^ Whitten, Kenneth W.; Gailey, Kenneth D.; Davis, Raymond East. (1992). General chemical science (quaternary ed.). Saunders Higher Publishing. p. 475. ISBN0-03-072373-6.
- ^ Boreyko, Jonathan B.; Hansen, Ryan R.; Irish potato, Kevin R.; Nath, Saurabh; Retterer, Scott T.; Collier, C. Patrick (2016). "Decision-making condensation and frost growth with chemical micropatterns". Scientific Reports. 6: 19131. Bibcode:2016NatSR...619131B. doi:ten.1038/srep19131. PMC4726256. PMID 26796663.
- ^ "Sublime". Dictionary.com Unabridged. Random House.
- ^ Fassnacht, South. R. (2004). "Estimating Alter-shielded guess snowfall undercatch, snowpack sublimation, and bravado snowfall transport at half dozen sites in the coterminous United states". Hydrol. Process. 18 (18): 3481–3492. Bibcode:2004HyPr...xviii.3481F. doi:10.1002/hyp.5806.
- ^ Caroll, J. (2014). Natural Gas Hydrates. p. 16. ISBN9780128005750.
- ^ Staff writer(south) (2015). "what solid go through sublimation?". National Science Foundation and UCSB School-Academy partnership. Retrieved thirteen Nov 2022.
- ^ Pavia, D. (2005). Introduction to organic laboratory techniques. pp. 781–782. ISBN978-0534408336.
- ^ Girard, James (2011). Criminalistics: Forensic Science, Law-breaking and Terrorism. Jones & Bartlett Learning. pp. 143–144. ISBN978-0-7637-7731-9.
- ^ R. B. King Organometallic Syntheses. Volume 1 Transition-Metal Compounds; Academic Printing: New York, 1965. ISBN 0-444-42607-8.
- ^ Harwood, Laurence M.; Moody, Christopher J. (1989). Experimental organic chemistry: Principles and Practice (Illustrated ed.). WileyBlackwell. pp. 154–155. ISBN978-0-632-02017-1.
- ^ Barrett, Francis (1815). The lives of alchemystical philosophers: with a critical catalogue of books in occult chemistry, and a option of the nearly celebrated treatises on the theory and practice of the hermetic art. Macdonald and Son for Lackington, Allen, & Co. p. 233.
- ^ DiBernard, Barbara (1980). Abracadabra and Finnegans wake. SUNY Printing. p. 57. ISBN978-0873953887.
- ^ Ripley, George (1591). Compound of Alchemy.
- ^ Gavezzotti, A. (1997). Theoretical Aspects and Estimator Modeling of the Molecular Solid State. Chichester: Wiley and Sons.
- ^ McDonagh, J. Fifty.; Nath; De Ferrari, Luna; Van Mourik, Tanja; Mitchell, John B. O. (2014). "Uniting Cheminformatics and Chemic Theory To Predict the Intrinsic Aqueous Solubility of Crystalline Druglike Molecules". Periodical of Chemical Information and Modeling. 54 (iii): 844–56. doi:x.1021/ci4005805. PMC3965570. PMID 24564264.
- ^ McDonagh, James; Palmer, David Southward.; Van Mourik, Tanja; Mitchell, John B. O. (17 October 2022). "Are The Sublimation Thermodynamics of organic molecules predictable?" (PDF). Journal of Chemical Information and Modeling. 56 (xi): 2162–2179. doi:10.1021/acs.jcim.6b00033. hdl:10023/11874. ISSN 1549-9596. PMID 27749062.
External links
-
 Media related to Sublimation at Wikimedia Commons
Media related to Sublimation at Wikimedia Commons
Source: https://en.wikipedia.org/wiki/Sublimation_%28phase_transition%29
Posted by: dennisalannow.blogspot.com



0 Response to "is sublimation a physical or chemical change"
Post a Comment